Hydrogen Peroxide
H2O2, otherwise known as hydrogen peroxide, is a transparent chemical compound that is commonly produced as a distilled liquid (Britannicacom, 2016). It is mostly used for bleaching various types of textiles and wood, manufacturing other chemicals, as rocket propellant, cosmetic use and medicinal purposes (Britannicacom, 2016). It can be found in rain, cleaning supplies, factories, mines and your local pharmacy (NZIC, n.d.) There are four steps in the manufacture of hydrogen peroxide. Those four steps are hydrogenation, filtration, oxidation and extraction (NZIC, n.d.)
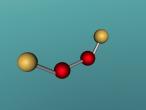
Hydrogen peroxide is composed of two hydrogen atoms and two oxygen atoms ( H2O2) that are in a covalent bond in which the two oxygen atoms share the two hydrogens’ electrons. This type of bond occurs when the outer most electrons of two or more atoms are shared in order to gain stability by forming a full electron shell (Chemistry LibreTexts, 2015). Oxygen, O, has an atomic number of 8 and can be found in group six of the periodic table. It has an electron configuration of 2 electrons on one inner shell and 6 electrons on the outer shell. Hydrogen, H, has an atomic number of 1 and can be found in group one of the periodic table. It has an electron configuration of 1 electron on its only shell. It is because of this bond that both oxygen atoms contain eight electrons on their outer electronic shells and are fully stable.
It is the simplest of peroxides. H2O2 decomposes into water and oxygen on heating or when it is exposed to various substances that includes the salts of metals. Pure hydrogen peroxide freezes at -0.43oC and has a boiling point of 150.2 oC (NZIC, n.d.). Physically, hydrogen peroxide is quite similar to water except for the fact that it is 40% denser. The bond between the two oxygen atoms is also quite weak so it is susceptible to fragmenting into different radicals. It is because of this that makes H2O2 a very effective oxidising agent.
Links to Expand Your Learning:
- https://www.britannica.com/science/hydrogen-peroxide
- https://en.m.wikipedia.org/wiki/Hydrogen_peroxide
Questions:
- Could H2O2 be used to make explosives because of its radical nature?
- How is used for medicinal purposes?
- How much hydrogen peroxide is to much for the human body?
There were several difficulties encountered during the programming of the hydrogen peroxide molecule. One such problem was creating the covalent bonds between the atoms. This was because they had to be angled and making contact with the atom in its centre.
References:
· Britannicacom. (2016). Encyclopedia Britannica. Retrieved 28 July, 2016, from https://www.britannica.com/science/hydrogen-peroxide
· New Zealand Institute of Chemistry (n.d.) The Manufacture of Hydrogen Peroxide. New Zealand:
- Chemistry LibreTexts (2015) Covalent Bonds – Chemistry LibreTexts. Retrieved 28 July, 2016 from http://chem.libretexts.org/Core/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds























Comments
Feedback
In-text?
Where are your atomic mass etc?