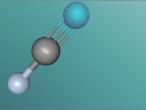
Hydrogen cyanide

Hydrogen cyanide, otherwise known as prussic acid is lethal to humans when a large enough quantity is ingested (approximately 150mg/m-3) and has a LD50 2857mg/kg of body weight. With a boiling point of 26°C and a melting point of 12-14°C, hydrogen cyanide is mostly found in its gaseous or liquid state, where it is most fatal as it is a systemic chemical asphyxiant where when the cyanide ion cyanide ion halts cellular respiration by acting as a non-competitive inhibitor for an enzyme in the mitochondria called cytochrome oxidase, halting ATP production in the mitochondria. However, hydrogen cyanide is a precursor to products from polymers to pharmaceuticals as it is then in small enough doses that there is no physical affect to the human body.
Hydrogen cyanide is a molecule where a hydrogen, carbon and nitrogen atom have bonded to form the toxic element, with one bond between the hydrogen and carbon and three between the carbon and nitrogen. While the hydrogen is not physically bonded with the nitrogen atom, the bonds connecting the hydrogen and the nitrogen atoms to the carbon atoms interlink, causing hydrogen to be in its divalent state, assuming that carbon is sp-hybridised (where the carbon atom/s is connected to all other atoms in the molecule) since it is joined to two other atoms.
Hydrogen cyanide is a very pale, blue transparent liquid or colourless gas, with a odour of almonds. Hydrogen cyanide is a good nucleophile due to it being a weak sour and a volatile compound. Due to this, when hydrogen cyanide is combined with water, it partially ionises to form CN-, more commonly known as cyanide. However, if a high enough ratio of hydrogen cyanide is combined with water, it will become hydrocyanic acid. As it is miscible in both water and ethanol, a high quantity of hydrogen cyanide would have to be combined with the water before hydrocyanic acid were to form.
Links for further information:
Structure of hydrogen cyanide molecule
http://www.chem1.com/acad/webtext/chembond/cb07.html
Characteristics of Hydrogen Cyanide
https://pubchem.ncbi.nlm.nih.gov/compound/hydrogen_cyanide
Extra information about Hydrogen Cyanide
https://www.quora.com/Is-hydrogen-cyanide-organic-or-inorganic
What was the coolest thing about hydrogen cyanide?
Did you know that whenever you eat apple seeds, you are basically eating cyanide?
When programming the hydrogen cyanide model, we wanted to make the bonds between the atoms half one colour and half the other colour. We accomplished this by having the size of one cylinder so that there would be enough space to put another coloured bond between the two atoms. We also found out that there would sometimes be internet crashes when programming so remember to always save your work every few minutes when you are coding as you don't want to have wasted all that time just to have to do it all over again.
Groups: