Ethanol Molecule

Ethanol
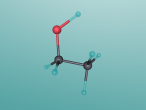
This blog post will be describing the structure and uses of the molecule ethanol. I will be going into detail about its composition, properties and applications of the molecule throughout the post. Ethanol is a molecule made up of nine atoms of three different types, being 2 carbon atoms, 1 oxygen atom and 6 hydrogen atoms. It has a molecular weight of 46.06844 g/mol, and it has the chemical formula of CH₃CH₂OH or C₂H₆O.
Hydrogen, one part of ethanol, is a non-metallic atom with the atomic number of 1 and symbol H. It is the lightest atom on the Periodic Table, and has the molecular formulae of H₂ when alone, as it only has one electron. Hydrogen is a very abundant atom on Earth, as it makes up 2 parts of water, which is where the majority of hydrogen is located.
Carbon, the second part of ethanol, is an atom with the atomic number 6 and symbol C. It is nonmetallic and has four electrons on the outer shell, with six in total. Carbon is used to form approximately 10 million compounds, making it present in a majority of all compounds in existence. It can be found in its pure form as either diamond or coal.
Oxygen, the final atom in ethanol, is an atom with the atomic number 6 and symbol O. It is non-metallic and is able to form oxides with many elements and compounds. It makes up 20.95% of the atmosphere, and is used in aerobic respiration in the human body.
Ethanol is a common form of alcohol, used for many things such as fuel, alcoholic drinks and antibacterial wash. It can be abbreviated to EtOH, and is part of the hydroxyl (-OH) and methylene (-CH2-) groups.
This was a difficult molecule to model, due to the fact that it contained 9 atoms, and the irregular structure it had. Because of this, the code used was rather messy, and could most likely be improved by more effective use of functions and more precise calculations. In addition, the different types of atoms proved difficult when creating the bonds, and as such they are rather basic in design.
Groups:
























Comments
Feedback
Good description but you have not provided links